Asfora Bullet Cage® System
Designed for rapid fusion with maximum versatility in interbody and instrument choices.
Indications:
The Asfora Bullet Cage® is indicated for spinal fusion procedures in skeletally
mature patients with degenerative disc disease (DDD) and instability in the lumbar spine at
one or two contiguous levels from L2 to S1. DDD for lumbar systems is defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies. The DDD patients may also have up to Grade I spondylolisthesis at the involved level(s). The ABC cage devices are used with autogenous bone graft. Patients should be skeletally mature and have had at least six (6) months of non-operative treatment prior to implant. When implanted via a posterior (PLIF, TLIF) approach, this device should be used with supplemental fixation.
Features of Asfora Bullet Cage® System
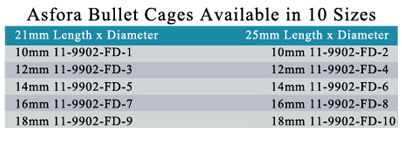
- Precision machined devices in a range of sizes to accommodate patient anatomy.
- Available in five diameters and two lengths.
- Sleek, modern bullet shaped design to facilitate easier insertion.
- Large cutting threads, angles toward the proximal end, allowing the device to be self-distracting and self-tapping.
- Threaded design enhances purchase to the vertebral endplates, decreasing the possibility of migration.
- Large slot aperatures allow for greater bone growth through the cage.
- Closed at both ends to prevent leakage of any fusion inducing material.
- Instrumentation for each approach is found in one complete set for maximum efficiency and ease of use.
- Instruments ergonomically designed with close attention to surgeon’s preferences and aesthetics.
- Extended length instruments available in a variety of sizes to accommodate a full range of anatomical differences.
- Sizes clearly etched on each instrument for easy identification.